Health
BioCryst Acquires Astria for $700M to Compete in HAE Market
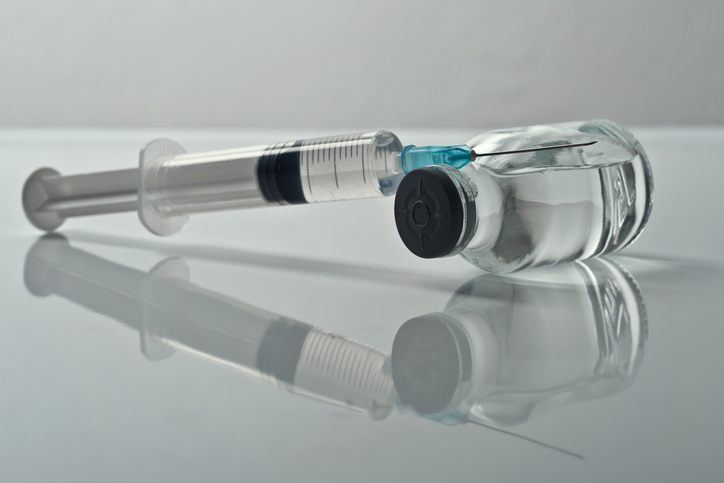
BioCryst Pharmaceuticals has announced the acquisition of Astria Therapeutics for approximately $700 million. This strategic move is aimed at enhancing BioCryst’s position in the competitive market for treatments of hereditary angioedema (HAE), a rare disease that causes severe swelling attacks. The acquisition will potentially provide patients with a new treatment option that could reduce the frequency of injections to as few as two per year.
Hereditary angioedema is characterized by unpredictable swelling episodes that can be life-threatening, especially when they affect the airway. Currently, patients have access to injectable medications that require administration every two weeks to every two months. BioCryst, based in Durham, North Carolina, already markets Orladeyo, a once-daily oral medication designed to prevent these attacks by inhibiting the kallikrein protein involved in the swelling process.
Strategic Acquisition and Future Prospects
Astria Therapeutics, headquartered in Boston, is developing a kallikrein inhibitor named navenibart, which could significantly improve patient compliance through less frequent dosing. The company has initiated a global Phase 3 clinical trial for navenibart, testing various dosing regimens, including a 600 mg dose followed by 300 mg every three months or 600 mg every six months. The primary endpoint of the trial is to evaluate the frequency of HAE attacks during the treatment period. Preliminary results are expected by early 2027.
In addition to its existing HAE product, BioCryst anticipates a decision from the U.S. Food and Drug Administration (FDA) regarding an oral granule formulation of Orladeyo for children aged 2 to 11 by December 12. The increase in competition within the HAE market is evident, as Takeda Pharmaceutical currently leads with its drug Takhzyro, approved in 2018. Takhzyro, administered every two weeks, has also seen newer alternatives gain traction, including Andembry from CSL Behring and Dawnzera from Ionis Pharmaceuticals.
BioCryst’s acquisition of Astria could allow the company to position navenibart as a leading injectable therapy. The potential for three- or six-month injection intervals is seen as a significant advantage over existing treatments. Early data from navenibart’s Phase 1b/2 trial demonstrated an average 92% reduction in HAE attacks, with a 50% attack-free rate, suggesting it may outperform current options.
Financial Implications and Future Growth
According to BioCryst’s Chief Commercial Officer Charlie Gayer, the market’s demand is not solely focused on increased efficacy but rather on reducing the burden of treatment. “What the market isn’t looking for is more efficacy; what it’s looking for is less burdensome dosing,” Gayer stated during a conference call on October 14. The company believes that navenibart’s less frequent dosing schedule, coupled with minimal injection-site pain, will attract patients seeking more convenient treatment options.
Orladeyo has been a significant contributor to BioCryst’s revenue, generating $437.6 million in sales last year, with expectations to reach $550 million by 2025. The company projects that its HAE portfolio could achieve revenues of $1 billion by 2029, eventually surpassing $1.8 billion by 2033.
The acquisition of Astria is structured as a combination of $8.55 in cash and 0.58 shares of BioCryst stock per share of Astria, valuing Astria at around $13 per share, a 53% premium over its previous closing price. BioCryst has secured up to $550 million in debt financing from funds managed by Blackstone, part of which will help fund the cash component of the acquisition. The transaction is expected to close in the first quarter of 2026, pending regulatory and shareholder approvals.
Upon completion, Astria CEO Jill Milne will join BioCryst’s board of directors, and Astria shareholders will hold approximately 15% of the combined company. BioCryst plans to explore strategic options for Astria’s other assets, including STAR-0310, which targets atopic dermatitis but does not align with BioCryst’s focus on rare diseases.
This acquisition marks a significant step for BioCryst as it seeks to expand its offerings in the HAE space and solidify its market position against larger competitors.
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Initiative to Monitor Disasters
-

 Lifestyle2 months ago
Lifestyle2 months agoToledo City League Announces Hall of Fame Inductees for 2024
-

 Business2 months ago
Business2 months agoDOJ Seizes $15 Billion in Bitcoin from Major Crypto Fraud Network
-

 Top Stories2 months ago
Top Stories2 months agoSharp Launches Five New Aquos QLED 4K Ultra HD Smart TVs
-

 Sports2 months ago
Sports2 months agoCeltics Coach Joe Mazzulla Dominates Local Media in Scrimmage
-

 Health2 months ago
Health2 months agoCommunity Unites for 7th Annual Walk to Raise Mental Health Awareness
-

 Politics2 months ago
Politics2 months agoMutual Advisors LLC Increases Stake in SPDR Portfolio ETF
-

 Science2 months ago
Science2 months agoWestern Executives Confront Harsh Realities of China’s Manufacturing Edge
-

 World2 months ago
World2 months agoINK Entertainment Launches Exclusive Sofia Pop-Up at Virgin Hotels
-

 Politics2 months ago
Politics2 months agoMajor Networks Reject Pentagon’s New Reporting Guidelines
-

 Science1 month ago
Science1 month agoAstronomers Discover Twin Cosmic Rings Dwarfing Galaxies
-

 Top Stories1 month ago
Top Stories1 month agoRandi Mahomes Launches Game Day Clothing Line with Chiefs







